New authorization requirements for rodenticides with anticoagulants
For rodenticides with anticoagulants, there is an important change in the authorization requirements in Switzerland:
- Due to the high risks for humans and the environment, the Notification Authority, in agreement with the assessment authorities, will no longer grant authorizations for rodenticides with anticoagulants for mice and rats for private users (general public, private individuals) from 1 April 2025. The use of all products with anticoagulants already authorized for private users will also be revoked from this date. After the transitional periods have expired, these products may no longer be sold to private users.
- There are currently no restrictions for professional users with or without a certificate, this is subject to any restrictions that may arise at the EU level during the second extension of anticoagulant active substances.
Rodenticides with anticoagulants
Rodenticides are products used to control mice and rats. If they are used for human or material hygiene protection purposes, they fall under the Ordinance on Biocidal Products (OBP, SR 813.12) and are subject to an authorization procedure. The Swiss and EU biocidal products regulations are harmonized (MRA 0.946.526.81) and the approvals of biocidal products are mutually recognized by Switzerland and EU countries.
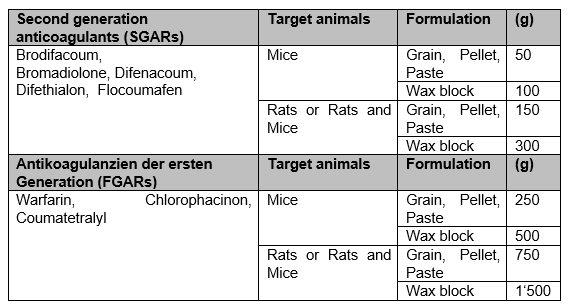
Most rodenticides that are available on the market as baits contain an anticoagulant as an active ingredient. These active ingredients are referred to as anticoagulants. Due to their high efficacy, second-generation anticoagulant rodenticides (SGARs; bromadiolone, difenacoum, brodifacoum, flocoumafen and difethialone) are primarily used. A single bait uptake is usually sufficient to achieve a lethal effect in rodents. In the case of rodenticides with first-generation anticoagulants (FGARs; warfarin, chlorophacinone, coumatetralyl), repeated bait uptake is necessary.
Anticoagulants are highly toxic to humans and animals. They are also poorly degradable in the environment and accumulate in living organisms. Consequently, they fulfil the exclusion criteria for being «persistent, bioaccumulative and toxic» (PBT). In addition, they are toxic to reproduction and, due to their anticoagulant mode of action, specifically toxic to the blood (toxic to target organs).
Due to their hazardous properties, the use of rodenticides with anticoagulants is only under exception circumstances eligible for authorization and the OBP requires that, as part of the comparative assessment, they should be replaced by less hazardous alternatives as soon as such become available. In the absence of alternatives that are similarly effective but at the same time less harmful to the environment and health, all uses of rodenticides were initially authorized under derogations (Art. 11e or Art. 12 para. 1 OBP). These exceptionally authorized uses were for the first time restricted in 2017 so that private users may only use such biocidal products indoors with the utmost caution and with all appropriate and available risk-reduction measures, which are specified in the authorization.
In view of their hazardous properties, in particular their high toxicity, the anticoagulant active substances are re-evaluated in the EU every five years. In addition, the authorization of the biocidal products is also limited to five years. The authorization requirements are set out in the Summary of Product Characteristics (SPC). The SPC document is a central part of a product authorization and also includes requirements specific to Switzerland.
New findings since the first renewal of the anticoagulant rodenticides in 2018
In 2022, a study (Rietgraf et al., 2022) was published showing that wildlife in Switzerland is also contaminated with anticoagulants. Residues of anticoagulants were found in many hunted foxes and birds of prey that had been involved in accidents, indicating secondary exposure (poisoned rodents as feed). However, the active substances were also found in hedgehogs and fish, indicating widespread contamination of the aquatic and terrestrial environment.
In 2022 and 2023, a series of questions for the comparative assessment, which is necessary for the second renewal of rodenticides with anticoagulants, were discussed at EU level. The answers were then published in the EU Commission's Implementing Decision (EU) 2024/816 in March 2024. The study cited in this decision concerning efficacy, as well as other studies submitted during the public commenting period on anticoagulant active substances, showed that snap traps are sufficiently effective for controlling mice indoors in private areas if these traps fulfil the criteria of the «NoCheRo-Guidance for the Evaluation of Rodent Traps» and private users are informed about the correct handling.
On the basis of these new findings, the Notification Authority, in agreement with the assessment authorities, has reviewed all authorizations of rodenticides with anticoagulants in accordance with Art. 23 para. 1 OBP.
New authorization requirements for private users (general public, private individuals) from 1 April 2025
The following considerations were taken into account:
- There are high risks of secondary poisoning for non-target organisms, i.e. poisoning due to the consumption of poisoned rodents. Unlike mice, rats do not remain indoors, but rather move freely from the inside to the outside and vice versa. As a result, rats mostly die outside and can be eaten by non-target organisms such as birds or foxes. Calculations of these secondary poisonings show unacceptable risks for these wild animals. The study published in 2022 (Riegraf et al., 2022) confirmed that a high number of wild animals tested in Switzerland are contaminated with anticoagulants. This study also indicates widespread contamination of the aquatic and terrestrial environment.
- According to the study cited in the Implementing Decision (EU) 2024/816 and other studies submitted during the public commenting period for the anticoagulant active substances, snap traps to control mice indoors are sufficiently effective for use in private households if these traps meet the criteria of the «NoCheRo-Guidance for the Evaluation of Rodent Traps» and private users are informed about the correct handling.
- Rodenticides with alternative chemical active substances for the control of mice will continue to be authorized for private users in indoor areas and can be used in accordance with the instructions for use.
- It is also possible to obtain expert advice and assistance in controlling mice and rats from professional pest controllers, which in most cases proves to be the safer and more efficient solution for rodent infestations in private homes. In particular, successfully controlling rats often requires structural or other measures that are difficult for private users to carry out. Therefore, the use of rodenticides with anticoagulants by private users to control rats alone leads to repeated use of highly toxic baits without achieving adequate results. This in turn greatly increases the risk of these baits being ingested by children and non-target organisms, which might cause poisoning.
In view of the new findings described above, the Notification Authority, in agreement with the assessment authorities, has come to the conclusion that the conditions for exceptionally authorizing the private use (Art. 11e or Art. 12 para. 1 OBP) are no longer met. The following restriction is therefore being implemented:
From April 1, 2025, no more authorizations will be granted for rodenticides with anticoagulants for mice and rats for private use. Consequently, from that date, the use by private users of all products with anticoagulants that are already authorized will be revoked. After the sales deadlines have expired, these products may no longer be sold to private users. The sales deadlines apply in accordance with Art. 26a VBP.
Until the expiry of the transitional periods, anticoagulant rodenticides may only be supplied to private users (general public, private individuals) in Switzerland under the following conditions:
- Use is exclusively possible in secured tamper-resistant bait boxes
- Use only inside buildings
- Loose bait may only be sold in sachets.
- Pulsed or permanent baiting is not allowed.
- Restrictions on package sizes:
Private users may only use rodenticides with an active ingredient content of less than 0.003%.
No changes to the authorization requirements for professional users for the time being
At present, no restrictions are planned for professional users with or without a certificate. However, this is subject to the EU Commission's implementing regulations for the approval of the second applications for the prolongation of the anticoagulant active substances. All restrictions introduced at the active substance level in the EU must also be implemented in the product authorizations in Switzerland.
The use of rodenticides with anticoagulants will continue to be authorized in accordance with the STOP principle (Art. 24a of Ordinance 3 to the Federal Act on Occupational Safety, SR 822.113) and the principles of integrated pest control in accordance with Art. 11e or Art. 12 para. 1 OBP.
For professional users without a certificate:
Until the second applications for the renewal of the anticoagulant active substances have been approved, the following provisions continue to apply to the product authorization of rodenticides with anticoagulants for professional users without a certificate in Switzerland:
- Use is exclusively possible in secured tamper-resistant bait boxes
- Use is only possible in and around buildings
- ·Pulsed or permanent baiting is not permitted
- No minimum package size is specified
Professional users without a certificate may continue to use rodenticides with an active substance content of ≥ 0.003%.
For professional users with a certificate of competence:
Until the second applications for the extension of the anticoagulant active substances have been approved, the following conditions continue to apply to the product authorization of rodenticides with anticoagulants for professional users with a certificate in Switzerland:
- Use only in secured tamper-resistant bait boxes or at hidden, inaccessible and protected bait points (the same level of protection for non-target animals and humans as with tamper-proof bait boxes) is possible
- Use in and around buildings, outdoors, in landfills and in sewers is possible
- Pulsed baiting only with products containing brodifacoum, flocoumafen or difethialone
- Permanent baiting is strictly limited to places with a high potential for reinfestation and when other control methods have been insufficient, only with products containing bromodialone or difenacoum
- No minimum package size is specified.
Professional users with a certificate may continue to use rodenticides with an active substance content of ≥ 0.003%.
Authorized products with alternative chemical active substances such as alphachloralose, cholecalciferol and carbon dioxide are not affected by the review and may continue to be used against rats and mice in accordance with the instructions for use.
Note the label and leaflet
Which uses are actually permitted for which user categories and which risk reduction measures are to be followed depends on the product. It is therefore important to always read the label, the leaflet and, if applicable, the safety data sheet carefully before using a product and to follow the instructions for use exactly.