Published on 8 April 2025
FAQ Unique Formula Identifier (UFI)
General
The purpose of the UFI is first and foremost rapid and clear identification of the chemical composition of a product in the event of an emergency call to Tox Info Suisse. Use the chemical composition of the product filed in the Chemicals Product Register (RPC), the necessary medical measures can be recommended by Tox Info Suisse and initiated by the treating physician.
The acronym UFI stands for “Unique Formula Identifier”.
The code is preceded by UFI in capital letters, followed by a colon “UFI:” then the alphanumeric 16-digit code, which is separated into four sections by hyphens.
Example: UFI: VDU1-414F-1003-1862.
All numbers between 0 and 9 may be used for the UFI. However, the letters B, I, L, O and Z may not be used to avoid confusion with other characters: B could be confused with 8; I and L could be confused with 1; O could be confused with 0 (zero); and Z could be confused with 2.
According to Art. 15a of the Chemicals Ordinance, the UFI, preceded by the acronym “UFI:” in capital letters, must be printed in a clearly visible, legible and indelible manner on the label of chemical products. It must also be recorded in the Chemicals Product Register (RPC) at the time of notification.
The Unique Formula Identifier (UFI) is a 16-character alphanumeric code that must be applied on the labels of chemical products that are classified as hazardous on the basis of their health and physical effects (H3xx or H2xx), and enables reference to the information on the composition of the product filed in the Chemicals Product Register (www.rpc.admin.ch).
Poison control centre Tox Info Suisse
Using the UFI, Tox Info Suisse can determine the precise composition of the product in the Federal Chemicals Product Register (RPC) and recommend the necessary medical measures.
Private and professional use of chemicals
In the event of poisoning or suspected poisoning, contact Tox Info Suisse immediately at 145. Using the UFI that you find on the label of the chemical, the expert at Tox Info Suisse can quickly look up the composition of the chemical in the Chemicals Product Register (RPC) and initiate the required medical measures, if necessary. However, in most cases, the consulting physicians will give the all-clear, meaning that medical measures are not necessary. In other cases, they will give consumers advice on first aid and tell them to go to a hospital, if required.
To protect employee health, when handling chemicals in the company, the employer must fulfil a duty of care in accordance with Art. 24a EmpO 3. The duty of care is described in various documents (see Factsheet [not available in English], Brochure [not available in English], Instructions [not available in English] and www.chematwork.ch [not available in English]). By way of support for implementing the duty of care, the SECO recommends use of the online tool SICHEM e.g., to create a chemical inventory. SICHEM is linked to the Chemicals Product Register (RPC, www.rpc.admin.ch). With the Unique Formula Identifier (UFI), SICHEM users can quickly and easily enter chemicals into their chemical inventory, as the product search function includes the UFI as one of the possible search criteria.
In the event of chemical poisoning, the doctor or Swiss poison control centre (Tox Info Suisse, emergency number: 145), should be given the UFI, if known and available, to ensure appropriate advice and treatment are received. To avoid cases of poisoning, the duty of care should be exercised when handling chemicals. This means that measures to protect employee health when handling chemicals in the company should be defined and implemented according to the STOP principle (c.f. Art. 24a EmpO 3). If companies handle hazardous chemicals, the ASA mandatory obligation to consult specialists according to Annex 1 of FCOS Directive no. 6508 [not available in English] applies.
Either the chemical is only classified as environmentally hazardous, as indicated by the GHS pictogram with the dead fish or the hazard statements for environmental hazards (e.g., H400 – Very toxic to aquatic life), or the chemical is classified as non-hazardous. In both cases, no UFI is needed on the label of the chemical. However, it may also be an older chemical (e.g., stock) that would be subject to the UFI obligation but was not yet labelled with a UFI.
Chemical products without a UFI that the customer has can be used until their expiry date and then disposed of appropriately. In an emergency, Tox Info Suisse can identify the chemical based on the trade name, provided that the product has been correctly notified in the Chemicals Product Register (RPC).
Placement of chemicals on the market
The product does not require a UFI but according to Art. 48 in combination with Art. 19 let. d no. 4 ChemO must be notified in the Chemicals Product Register (RPC).
Yes, fuels must be labelled with a UFI and this must be notified in the Chemicals Product Register (RPC).
A distributor who received, for example, products without a UFI in June 2025 because they did not yet have to have a UFI at that date may continue to sell them without a UFI after 1 January 2026. The obligation to provide a product with a UFI lies with the manufacturer or importer. The sale of products without a UFI is not currently regulated by law.
The importer is also a manufacturer within the meaning of Art. 4, para. 1, let. f, ChemA and is therefore required to provide products that must have a UFI by 1 January 2026 at the latest and to declare this in the chemicals register.
Art. 54 para. 2 let. b ChemO applies to such preparations
2 The following are not exempt from the notification obligation according to this chapter:
b. Preparations according to paragraph 1 letters b, c, h, i and j that have a UFI.
This means that the notification obligation including UFI still applies.
The principle applies that where there is a UFI, the product including UFI and composition must also be recorded in Chemicals Product Register (RPC).
Yes, an importer can import products without a UFI, but must then assign them a UFI before placing them on the market. The obligation to assign a UFI to a product lies with the manufacturer or importer and must be complied with by 1 January 2026 at the latest.
Preparations, biocides, plant protection products, fertilisers and tobacco products (liquid refills according to Art. 24 & 27 TabPO (e-liquids)), that are not classified as hazardous on the basis of their health and physical effects may be labelled with a UFI voluntarily. According to Art. 54 para. 2 let. b ChemO, the UFI must then also be recorded in the Chemicals Product Register (RPC) and the responsible regulatory authority must be notified. The principle applies that where there is a UFI, the product including UFI and composition must also be recorded in RPC.
According to Art. 4 para. 1 letter c ChemO, a preparation, composition, mixture or solution consists of two or more substances. This includes certain diluted acids and bases that are listed with the notation ‘... %’ in Annex VI of the CLP Regulation.
Substances in aqueous solutions are therefore considered to be preparations and, if they are classified as hazardous due to their physical or health effects, fall within the scope of Article 15a of the ChemO and must be labelled with a UFI.
No, according to the Chemicals Ordinance, batteries are considered to be objects. No UFI has to be generated for them and they do not have to be notified.
Bespoke paints formulated in limited quantities for an individual consumer or professional user at the point of sale by tinting or mixing colours, are exempt from the notification obligation provided that:
- the UFIs of the added colourants subject to UFI requirements are indicated on the label of the base colour, if they constitute more than 0.1% of the final product. If the concentration exceeds 5%, they are also to be listed as a constituent (this corresponds to Art. 25 para. 8 of the CLP-Regulation); or
- the colourants are indicated in the notification of the base colour in the maximum concentration in which they are added. In this case, the paint may be sold with the label of the base colour.
-> See point 5.7 at the following link.
Concrete, plaster and cement are exempted from the notification obligation if the following two conditions are met:
1. They conform with any of the standard formulas specified in Part D of Annex VIII to the CLP Regulation and
2. They bear the UFI required by the Notification Authority.
-> See point 5.8 at the following link.
For products that do not have a UFI because they are no longer manufactured, it is necessary to check where they are located. If they are already on the market, they can continue to be sold without a UFI. In the event of poisoning, ToxInfo can also provide advice based on the hazardous substances contained in the product (provided, of course, that the product has been correctly declared).
If the products are still at the manufacturer's or importer's premises, for example in a warehouse, they must be provided with a UFI.
The mixture is subject to the notification obligation if it contains nanomaterials with biopersistent fibres or tubes with a length of more than 5 µm (Art. 48 ChemO). If the mixture does not pose any physical or health hazards, no UFI must be generated or declared on the label.
All mixtures classified as hazardous in a multi-component product require their own notification in the Chemicals Product Register (RPC) and their own UFI. As a rule, the UFI should appear on the label or packaging of all mixtures that are part of the end product. Multi-component notifications must be entered in the RPC via KIT (product on the market) and the components as “Components of a KIT”.
In Switzerland, the Unique Formula Identifier (UFI) is being introduced for preparations, biocides, fertilisers and tobacco products (liquid refills according to Art. 24 & 27 TabPO (e-liquids)) that are classified as hazardous on the basis of their health and physical effects.
- From 1 January 2022: preparations, biocidal products, fertilisers and tobacco products (liquid refills according to Art. 27 TabPO (e-liquids)) that are newly placed on the market, which are intended for private users.
- From 1 January 2022: preparations, biocides, fertilisers and tobacco products (liquid refills according to Art. 27 TabPO (e-liquids)) that already have a UFI. This category includes in particular, products imported from the EEA.
- From 1 January 2026: all other preparations, biocides, fertilisers and tobacco products (liquid refills according to Art. 27 TabPO (e-liquids)) that are classified as hazardous on the basis of their health and physical effects.
It is envisaged that plant protection products that are not yet labelled with a UFI when the fully revised Plant Protection Products Ordinance is scheduled to come into force on 1 December 2025 must be labelled with a UFI by 1 December 2027 at the latest.
No UFI needs to be generated for preparations (Art. 15a ChemO), biocides (Art. 38a OBP), fertilisers (Art. 44 para. 4 Fertilisers Ordinance), plant protection products (Art. 171 E-PlantPPO, in force from 01.12.2025) and tobacco products (liquid refills (e-liquids), Art. 24 & 27 TabPO)), which, for example, are not hazardous or are only classified as environmentally hazardous. In addition, no UFI is necessary for preparations that fulfil the requirements of Art. 54 para. 1 ChemO, i.e., do not fall under the obligation to notify.
Although there is no such obligation, products bearing a UFI must always be notified by the manufacturer or importer in the Chemicals Product Register (RPC). The authorisation (biocides, plant protection products) or licence (fertiliser) applies irrespective of the UFI obligation.
No UFI needs to be generated for substances, irrespective of whether they are classified as hazardous or not.
In both the EU and Switzerland, a UFI must be generated for all preparations, biocides, fertilisers, plant protection products and tobacco products (liquid refills according to Art. 24 & 27 TabPO (e-liquids)), that are classified as hazardous on the basis of their health and physical effects (H3xx or H2xx). This concerns both products for professional and private users. It is envisaged that plant protection products that are not yet labelled with a UFI when the fully revised Plant Protection Products Ordinance is scheduled to come into force on 1 December 2025 must be labelled with a UFI by 1 December 2027 at the latest.
The responsible distributor (Swiss manufacturer, Swiss importer or Swiss authorisation holder) can generate a UFI for preparations, biocides, fertilisers, plant protection products and tobacco products (liquid refills according to Art. 27 TabPO (e-liquids)) that are only placed on the market in Switzerland and not in the EEA using their Swiss VAT number. The UFI must then be recorded together with the composition of the product in the Chemicals Product Register (RPC) or stated in the application documents for biocides, plant protection products and fertilisers requiring a licence. For products whose composition has already been recorded in RPC, it is sufficient to add the UFI to the RPC and then requalify the notification by submitting it. For fertilisers, this process is only possible for fertilisers registered after the Fertilisers Ordinance entered into force on 1 January 2024. With the exception of fertilisers and plant protection products, changes can also be made using the mass reporting interface (MMT).
A UFI for preparations, biocides, fertilisers, plant protection products and tobacco products imported from the EEA is also valid in Switzerland and must be recorded by the Swiss importer together with the composition of the product in the RPC or stated in the application documents for biocides, plant protection products and fertilisers requiring a licence.
There is also the possibility of the chemical manufacturer of the product located abroad notifying the composition directly as a sub-user in the RPC without the main user (in this case the Swiss importer) being able to view the confidential data on the full composition.
Mixtures that are not themselves classified as a physical or health hazard but that according to Annex II Parts 1 and 2 of the CLP Regulation are subject to additional labelling requirements (e.g., EUH208) are subject to the notification obligation in Switzerland but not in the EU and do not require a UFI.
Packaging and labelling of chemicals
Generally speaking, it is not mandatory to list the UFI in the material safety data sheet for “packaged products”, but it is strongly recommended. In this case, the UFI must be given in Section 1.1 “Product identifier” of the material safety data sheet.
According to Art. 15a para. 3 ChemO, the UFI, preceded by the acronym “UFI:” in capital letters, must be printed or applied in a clearly visible, legible and indelible manner on the label of chemical products.
a. on the label in the section for further information according to Article 25 of the EU CLP Regulation; or
b. on the inner packaging together with the other labelling elements; (if the inner packaging is of such a nature or so small that the UFI cannot be printed on or applied to it, it may be printed on or applied to an outer packaging together with the other labelling elements).
For products that are not packaged (loose deliveries) and supplied to professional users, the UFI must be given in the material safety data sheet (Section 1.1 Product identifier) or in the case of fertilisers, in the accompanying documents (Annex 3 of the Fertilisers Ordinance).
For non-packaged products that are supplied to private users, it is possible to include the UFI in the copy of the labelling elements (Art. 15 a para. 4 ChemO).
Importing chemicals from the EU into CH
The Swiss Chemicals Act does not recognize industrial use. Therefore, according to Art. 15a of the Chemicals Ordinance (ChemO), the UFI must be indicated on the label (or packaging) and not in the SDS. However, chemical products (substances and preparations) that are legally compliant on the market in an EU or EEA can be placed on the market in Switzerland under the Cassis-de-Dijon principle (CdD principle). Biocidal products, plant protection products and new substances subject to notification are not covered by the Cassis-de-Dijon principle. Further exceptions that do not fall under the CdD principle can be found here.
This means, that preparations for industrial use, for which the UFI is not indicated on the label (or packaging) but in the SDS, may be placed on the market in Switzerland in accordance with Art. 16a of the Federal Act on Technical Barriers to Trade (THG). However, these chemicals including the UFI must be reported by the responsible Swiss importer to the Product Register for Chemicals (RPC). As Article 16b THG prohibits discrimination against domestic manufacturers, the regulation also applies to Swiss manufacturers.
The UFI from the EU can be used in Switzerland and must be recorded together with the current composition in the Chemicals Product Register (RPC). This is the only way to establish the connection between a product, UFI and composition in the event of poisoning.
There is also the possibility of the chemical manufacturer of the product located abroad notifying the composition directly as a sub-user in the RPC without the main user (in this case the Swiss importer) being able to view the confidential data on the full composition.
UFIs generated for the EEA for the current composition can also be used to notify the product in the Chemicals Product Register (RPC).
Exporting chemicals from Switzerland
No, UFIs generated in Switzerland are not recognised in the EU due to the lack of a bilateral agreement.
No, a UFI is not mandatory for chemicals being exported to the UK. Importers and manufacturers based in the UK who supply the British market are encouraged to voluntarily provide information relevant to the provision of healthcare and on preventive measures. In practice, this is done by submitting the material safety data sheet (MSDS) by e-mail to the National Poison Information Service (NPIS). There is no obligation to provide a UFI, although it can also be registered if generated. It must also be shown clearly on the first page of the submitted material data safety sheet in Section 1.1 “Product identifier”.
Biocides
For biocides that require a Unique Formula Identifier (UFI), the UFI must already be submitted when applying for authorisation. If the UFI is not yet available when the application is submitted, it must be provided to the Notification Authority for Chemicals at least 30 days before the product is first placed on the market. In practice, this is done by independently entering the UFI in the “Composition” section of the dataset in the Chemicals Product Register (RPC) at www.rpc.admin.ch.
Please note that the product is not automatically released or qualified in the RPC after the UFI has been recorded and the notification has been submitted. Rather, it is given the status “under examination”. Please therefore inform the Notification Authority for Chemicals of the change by sending an e-mail to cheminfo@bag.admin.ch.
Plant protection products
It is envisaged that plant protection products not yet labelled with a UFI when the fully revised Plant Protection Products Ordinance is scheduled to come into force on 1 December 2025 must be labelled with a UFI by 1 December 2027 at the latest.
Fertilisers
Fertilisers that were notified or licensed before 1 January 2024 (under the old legislation):
Recording the UFI in the Chemicals Product Register (RPC) represents a change to the data and the labelling of the fertiliser. According to the transitional provisions in Art. 44 para. 2 and 3 of the Fertilisers Ordinance, any change to the fertiliser or its labelling requires the fertiliser to be registered, or a new licence application must be submitted in accordance with the new provisions. Specifically, the user must transfer the data for the fertiliser from the old to the new version of the fertilisers section of the RPC. The transfer is automatic. If the user wants to change some information, the system asks whether it should migrate the data to the new user interface. However, not all data required in the new version are available in the old version (product function categories (PFC), component material categories (CMC) of the raw materials, etc.). After the transfer, the user has to add the missing data and complete the registration process or submit the application for authorisation for evaluation.
Fertilisers licensed after 1 January 2024 (under the new Fertilisers Ordinance):
Entering the UFI number in the Chemicals Product Register (RPC) represents a change to the data and the labelling of the fertiliser. In addition to entering the UFI number, the users must upload a new label (that includes the UFI number). They must then submit the application to the FOAG for evaluation. If no further changes are made in this case, we recommend making a comment in field no. 12 that the change only concerns the UFI number. The FOAG can then directly qualify the fertiliser without issuing a new licence (the UFI number is not listed on the licence).
Fertilisers registered after 1 January 2024 (under the new Fertilisers Ordinance):
The user must enter the UFI number in the Chemicals Product Register (RPC) and replace the label in the documentation. As the registration process is independent (the FOAG does not evaluate the details of the fertiliser), the importer/manufacturer (Swiss company) must complete the registration after the changes are made. They must also resubmit the fertiliser to publish the details.
UFI generator
To generate a Swiss UFI, you need the company's VAT ID number and a formulation number specific to the composition or individual notification concerned. If you enter both of these numbers in the Swiss UFI generator, you will get a corresponding Swiss UFI. The VAT ID number ensures that the UFI is unique and there are no overlaps with the Swiss UFIs of other companies. Most companies probably already use internal formula codes. If these are exclusively numerical and the numbers range from 0 to 268 435 255, they can be used directly as the formulation number in the Swiss UFI generator. In all other cases, e.g., if the codes are alphanumeric or include other characters, you must first assign new formulation numbers with the correct format to your mixtures. You may not use a combination of formulation number and VAT ID number more than once if the composition of the mixtures differs. We also recommend filing the combination of composition and selected identification number internally.
No. The Swiss UFI generator and the Chemicals Product Register (RPC) are independent from one another. A UFI generated using the Swiss UFI generator must subsequently be entered manually or via MMT in the RPC.
Yes, in such cases the federal authorities recommend using the ECHA UFI generator. It allows the generation of a UFI without a VAT number and will also be recognised in Switzerland. As the ECHA generator does not allow a UFI to be generated using a Swiss VAT number, a Swiss generator has been made available (which only works with a Swiss VAT number).
The algorithm of the Swiss UFI generator always generates a unique code based on entering a VAT number and the formulation number; this code is not saved by the Swiss UFI generator. You can therefore consistently reproduce and copy the code by entering the same parameters – however, we recommend filing and, if necessary, maintaining (as the formulation number changes for each composition) the UFIs generated in your database.
The EU (UFI generator) and the Swiss UFI generator (UFI (Unique Formula Identifier)) are IT applications which can generate a valid, marketable UFI using coding developed by the EU (format: xxxx-xxxx-xxxx-xxxx, alphanumeric). Generally speaking, three elements are required for a UFI:
- The country in which the company is headquartered
- The company's VAT number (as well as its UID number in Switzerland) and
- A formulation number for the mixture (i.e. composition)
Both generators operate independently and are not linked to other applications (such as the Chemicals Product Register (RPC) in Switzerland). One or more UFIs (batch) can be generated in a session. Companies are responsible for generating and managing the UFIs of their products.
Unlike the Swiss UFI generator, the EU UFI generator contains an additional validation function. This involves checking whether the UFI has been created correctly in accordance with the generator's requirements and if it is marketable in this form. The identical combination of country, VAT and formulation numbers always leads to the same UFI. However, this differs based on whether the EU or the Swiss UFI generator is used.
As Switzerland is not an EU member state, the ECHA UFI generator cannot generate a UFI with a Swiss VAT number. Switzerland therefore has to provide its own unique Swiss UFI generator (UFI (Unique Formula Identifier)), but this only works with a Swiss VAT number. The UFI generated with the Swiss UFI generator only applies in Switzerland.
Revising the UFI
Article 52 of the Chemicals Ordinance (ChemO) outlines when an existing notification in the RPC has to be updated by the responsible distributor.
The notification in the Chemicals Product Register (RPC) must be updated in the event of:
- any modifications to the information specified in Articles 49 and 50 ChemO; these must be notified within three months.
- changes to classification and labelling.
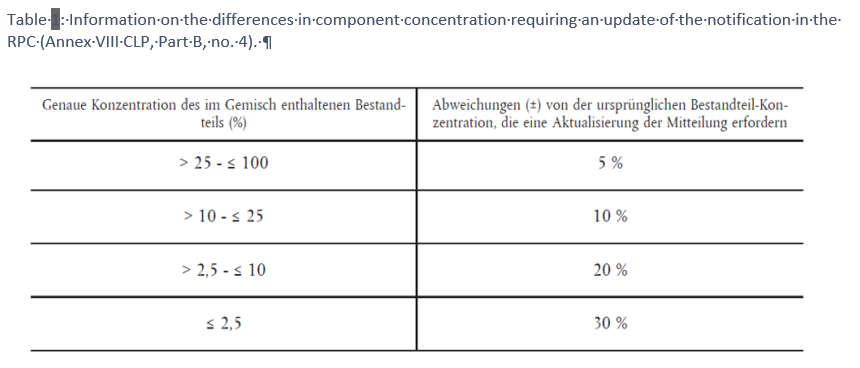
- changes to the concentration of components according to the provisions of Annex VIII CLP, Part B no. 4 (Tab. 1).
If the composition needs to be updated according to these provisions, a new UFI must also be generated, specified in the existing notification and applied to the product.
Note: While ChemO refers to Annexes I to VI of the EU CLP Regulation, it does not refer to Annex VIII. To avoid trade barriers, it makes sense to apply the same rules for updating the composition as in Annex VIII of the EU CLP Regulation.
The UFI in the Chemicals Product Register (RPC)
No. The PCN notification does not contain all information that is required in Switzerland and e.g., recorded as mandatory fields in the Chemicals Product Register (RPC). Even if the PCN were accepted, the company would have to revise and possibly add to each individual entry after import (manually or via mass reporting interface (MMT)). The differing data structures and maintenance concepts (e.g., substance imports via REACH, MiM, etc.) are a further problem.
No. The UFI is indicated on the label and in some cases is part of the material safety data sheet. It is therefore not confidential information according to Art. 73 ChemO. The UFI is published in the Chemicals Product Register (RPC).
In addition to the author of the full composition identified in the Chemicals Product Register (RPC), Tox Info Suisse and the Federal authorities have access to this information. Confidential data on the composition may also be made available to the cantonal authorities for the purpose of reviewing the UFI (Art. 75 para. 5 ChemO). See also the definition of authorship (Authorship).
Yes, the UFI is integrated as a data point in the mass reporting interface (MMT) and can also be updated. Additional information on the MMT and access requirements are available here: Mass reporting interface (MMT).
The PCN format (IUCLID file) from the ECHA notification tool cannot be entered directly into the Chemicals Product Register (RPC). The main reasons for this are that
- the file format cannot be read into the RPC,
- the information content for EU notification is not fully harmonised with Switzerland, and
- the underlying PCN-RPC IT structure differs greatly (e.g., Swiss chemicals legislation does not recognise substance registrations according to REACH or the EEA supply chains).
As part of the introduction of UFIs in Switzerland, the Notification Authority for Chemicals is offering companies the option of a one-off bulk import for newly generated UFIs (from 25+) for initial allocation to their chemical products. The requirements for this are:
- no use of the MMT
- at least 25 UFIs
- 1x per company, after which independent entry and maintenance in the RPC by relevant users (self-monitoring)
The following documentation must be submitted:
- Send an Excel file with CPID number, product name and associated UFI to cheminfo@bag.admin.ch.
Note: Processing time of approx. one month after receipt of the full documentation should be expected.
Changes to existing UFIs in the RPC must be made by the notifier on receipt of the product history. Future allocations of and any updates to UFIs must then be made according to the self-monitoring process.
Nel Registro dei prodotti chimici (RPC), solo l’UFI della composizione corrispondente può essere registrato nell’annuncio. Le composizioni etichettate come MiM devono essere chiaramente segnalate dall’annunciante (p. es. mediante inclusione del relativo annuncio qualificato).
No. Although the details of a UFI are saved in the database with the composition concerned when fulfilling the notification obligation for a preparation in the Chemicals Product Register (RPC), they are not available for further notifications for reasons of confidentiality and responsibility.
The mass reporting interface simplifies the automatic transfer (via import) of a company's products (preparations, existing substances, biocides) to the Chemicals Product Register (RPC) via an XML file, greatly reducing transfer times and errors associated with manual entry. The MMT cannot be used for fertilisers and plant protection products. The company concerned is responsible for developing the software to create the XML file using the published user manual and codes.
Yes, for marketing reasons more than one UFI is possible and permitted for a composition. However, these must all be notified in the Chemicals Product Register (RPC). As the UFIs are published in the public section of the RPC, it should be noted that this approach may result in inadvertent conclusions. If disclosure and comparability of several UFIs in a single notification is not desired, the Notification Authority for Chemicals recommends individual notifications (i.e. one notification per trade name).
No, the details of a UFI in the Chemicals Product Register (RPC) are not automatically linked with an existing composition with the same UFI in the database. However, the duplication option can be used to input data more quickly.
Just like the accompanying change to the composition, a UFI can be updated directly in the product notification in the Chemicals Product Register (RPC), as the RPC has UFI versioning. However, this version is currently only released to the authorities, including Tox Info Suisse. Tox Info Suisse and the authorities can retrieve all UFIs (i.e. current and obsolete) via UFI search in the RPC and view the corresponding composition.
Yes, for marketing reasons more than one UFI is possible and permitted for a composition. However, these must all be notified in the Chemicals Product Register (RPC). As the UFIs are published in the public register, it should be noted that this approach may result in inadvertent conclusions. If disclosure/comparability of several UFIs in a single notification is not desired, individual notifications should be made (one notification per UFI).
As the Chemicals Product Register (RPC) continuously versions changes in the background, a new UFI with a new composition can be implemented in the same product.
For hazardous preparations, the notification of the composition according to Art. 49 para. 1 let. d no. 2 of the Chemicals Ordinance (ChemO) includes information on the components via the material safety data sheet (hazard-determining components). For hazardous preparations available to private users, the full composition must always be notified (Art. 50 ChemO). Components that are not hazardous according to Article 3 ChemO can be labelled with a name that identifies the most important functional groups.
For plant protection products, biocides and fertilisers requiring a licence, under the authorisation/licensing process, full details of the composition or the raw materials must always be given.
The current, marketable UFI must be notified in the “Composition” section. However, for fertilisers, the current, marketable UFI must be saved in the “Raw materials and component material categories (CMC)” section. Unlike the composition, the UFI details are not confidential information and are published in the public register.
The UFI must be indicated on the label and, together with the details of the product composition or the raw materials in the case of fertilisers, notified in the Chemicals Product Register (RPC). This is the only way to establish the connection between a product, UFI and composition in the event of poisoning. All products for which a notification is made using the same UFI must have the same composition.
Index
- General
- Poison control centre Tox Info Suisse
- Private and professional use of chemicals
- Placement of chemicals on the market
- Packaging and labelling of chemicals
- Importing chemicals from the EU into CH
- Exporting chemicals from Switzerland
- Biocides
- Plant protection products
- Fertilisers
- UFI generator
- Revising the UFI
- The UFI in the Chemicals Product Register (RPC)